Post-Marketing Studies & Real-World Evidence Clinical Trials
Your trusted partner for decentralized post-marketing surveillance clinical trials and real-world evidence collection. Point-of-need accessibility. Proven compliance.
.jpg?width=1100&name=Section2%20Image%202%20(1).jpg)
Traditional fixed-location sites face persistent challenges in post-marketing surveillance and post-approval studies. Limited patient access slows real-world data capture, while travel burden and scheduling constraints reduce retention and compromise patient safety monitoring. High operational costs and limited flexibility also make it difficult to generate evidence-based insights across diverse populations, which regulatory bodies such as the U.S. Food and Drug Administration (FDA) increasingly expect for ongoing safety studies.
|
Low Patient Compliance Patients often skip follow-up visits due to travel inconvenience, reducing adherence, study consistency, and overall long-term data reliability. |
Limited Real-World Data Restricted geographic reach limits diverse population representation, weakening study validity, generalizability, and real-world insight into treatment response, side effects, and product safety. |
High Dropout Rates Traditional trials experience 30–40% dropout in long-term studies, reducing statistical power, delaying timelines, and putting post-marketing commitments for new drugs at risk. |
Delayed Data Collection |
Why Choose 20/20 Onsite for Post-Marketing Surveillance Clinical Trials?
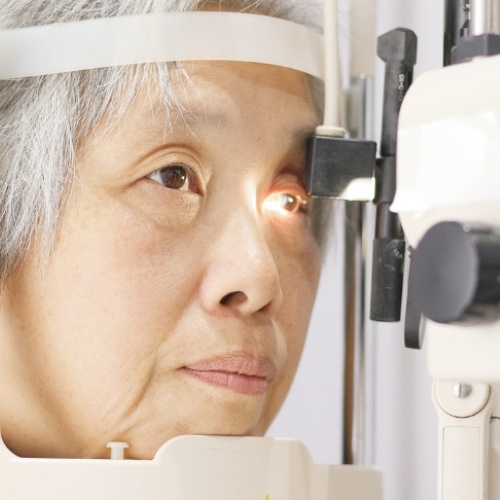
Post-marketing surveillance clinical trials are essential for monitoring drug safety and efficacy after FDA approval. At 20/20 Onsite, we revolutionize traditional post-marketing studies by bringing comprehensive ophthalmic assessments directly to patients' preferred locations, eliminating barriers that typically reduce participation and data quality in real-world evidence studies.
Our decentralized approach addresses critical challenges facing pharmaceutical sponsors: low patient compliance, limited geographic reach, and high dropout rates that compromise study integrity. By delivering point-of-need clinical services to homes, workplaces, and community centers, we ensure superior patient retention while capturing authentic, real-world data.
This innovative model enables sponsors to meet post-marketing commitments more efficiently, reduce operational costs, and generate higher-quality real-world evidence that supports regulatory submissions and market access strategies.
.webp)
Point of Need
100%
Screening timelines met across all business operations.
Data Integrity
98%
Data accuracy submission rate.
Patient Retention
91%
Patient retention rate vs industry standard 70%.
Patient Centricity
95+
Patient Net Promotor Score vs industry average NPS of 30-50.
Our Post-Marketing Surveillance Services
Our comprehensive approach supports FDA post-marketing surveillance requirements, with Phase IV clinical trial services and post-approval drug safety monitoring that help sponsors meet market authorization obligations across diverse therapeutic areas.
Study, Design, & Planning
- Target population analysis for real-world evidence studies
- FDA post-marketing requirements and commitments (PMR/PMC) planning
- Post-marketing surveillance clinical trials protocol development
- Site selection for decentralized post-marketing surveillance
Implementation & Data Collection
- Multi-site post-market clinical research execution
- Point-of-care surveillance and real-world data collection
- Digital pharmacovigilance studies platforms
- Flexible assessment scheduling for clinical trial post-marketing studies
Data Reporting
- Ongoing real-world evidence studies monitoring
- Post-approval clinical studies data reporting
- Regulatory reporting and safety signal detection
- Long-term outcome assessment and intervention
- FDA Sentinel Initiative safety monitoring compliance
Observational Study: Eye Assessments in Mobile Settings Have
the Same Results as In-Office Exams
Study Design & Planning
Clinical Trial Post-Marketing Studies with Comprehensive Protocol Development and Strategic Planning
Effective real-world evidence studies begin with a thorough study design and planning, yet many post-marketing surveillance clinical trials face challenges due to inadequate protocol development and population analysis.
Traditional planning approaches often overlook critical factors like real-world patient populations, geographic diversity, and regulatory compliance requirements that impact decentralized post-marketing surveillance success.
How Our Clinical Research Support Services Help?
20/20 Onsite provides comprehensive study design and planning services that establish the foundation for successful post-market clinical research through detailed target population analysis and feasibility studies for real-world evidence studies.
Our experienced team develops optimized clinical trial post-marketing studies strategies tailored to your specific post-approval requirements, including site selection for geographic diversity and regulatory compliance assessment for all post-marketing surveillance materials.
Read Case StudyImplementation & Data Collection
Post-Marketing Surveillance with Multi-Site Execution and Real-World Data Capture
During the implementation phase, post-market clinical research requires efficient data collection across diverse patient populations in real-world settings, yet many studies struggle with traditional site limitations and fragmented data collection approaches.
Traditional collection methods often rely on fixed-site approaches that miss key patient segments, particularly when real-world evidence studies require specialized populations or point-of-care surveillance capabilities.
How Our Clinical Research Support Services Help?
20/20 Onsite executes comprehensive multi-site clinical trial post-marketing studies that combine community outreach, physician networks, and digital pharmacovigilance studies platforms for maximum real-world data collection efficiency.
Our post-marketing surveillance clinical trials team coordinates point-of-care surveillance and flexible assessment scheduling, bringing decentralized post-marketing surveillance directly to participants through mobile capabilities and community partnerships.
Check Our Clinical Trial SolutionsData Reporting
Post-Marketing Surveillance with Comprehensive Regulatory Reporting
Maintaining data integrity and regulatory compliance throughout post-marketing surveillance clinical trials is critical for safety monitoring and regulatory requirements, yet data reporting challenges remain significant across all real-world evidence studies.
Traditional approaches often fail to address the complex data integration and safety signal detection requirements that are essential for effective pharmacovigilance studies and post-approval clinical studies.
How Our Clinical Research Support Services Help?
20/20 Onsite delivers ongoing real-world evidence studies monitoring through comprehensive safety signal detection and regulatory reporting that keeps post-market clinical research compliant throughout the study.
Our clinical trial post-marketing studies specialists provide dedicated support and regulatory reporting coordination, with point-of-care surveillance capabilities that ensure continuous data quality and safety monitoring.
Our post-marketing surveillance capabilities include comprehensive regulatory support for FDA compliance
We provide pharmacovigilance outsourcing post-approval with expertise in FDA post-marketing surveillance requirements.
|
Struggling With Post-Marketing Patient Compliance? Maintain patient engagement in long-term post-marketing surveillance clinical trials by making follow-up ophthalmic assessments more convenient and accessible through point-of-need services. |
Need Support with Real-World Evidence Collection? Ensure patients participating in post-marketing studies who require ongoing safety monitoring stay compliant with their scheduled follow-up appointments and data collection requirements. |
Trying to Meet FDA Post-Marketing Commitments? We ensure that the post-marketing surveillance experience delivers comprehensive regulatory compliance by providing specialized clinical personnel to conduct these assessments with appropriate documentation and accessibility. |
Industries We Serve for Post-Marketing Studies
|
Pharmaceutical Companies
|
Medical Device Manufacturers
|
Biotechnology Firms
|
Benefits of Our Post-Marketing Study Services
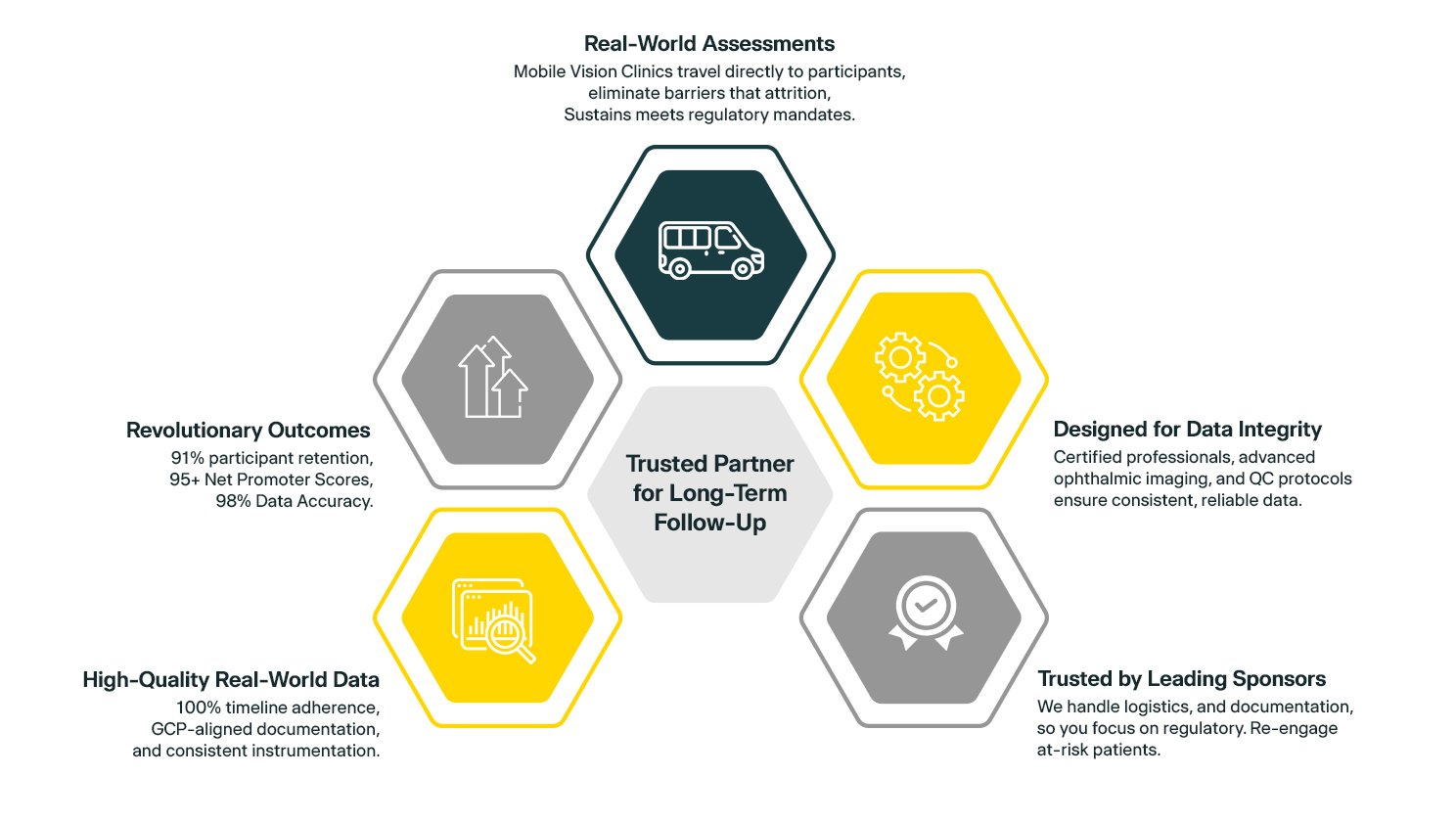
1. Trusted Partner for Long-Term Follow-Up20/20 Onsite handles all necessary logistics for post-marketing surveillance clinical trials point-of-need assessments, including travel, setup, exams, data submission and real-time technical support. With over 85,000 eye exams performed, 40+ clinical trials supported, and an average of 11+ years of ophthalmic experience per clinical professional, we deliver long-term ocular follow-up anywhere in the U.S.
2. Real-World Assessments Without the Drop-Off
Our Mobile Vision Clinics travel directly to participants for real-world evidence studies, eliminating barriers that cause attrition. Post-marketing surveillance clinical trials benefit from our national fleet, bringing protocol-specific eye exams wherever participants are located, sustaining engagement, and meeting regulatory mandates without sacrificing convenience.
3. Designed for Data Integrity in the Real World
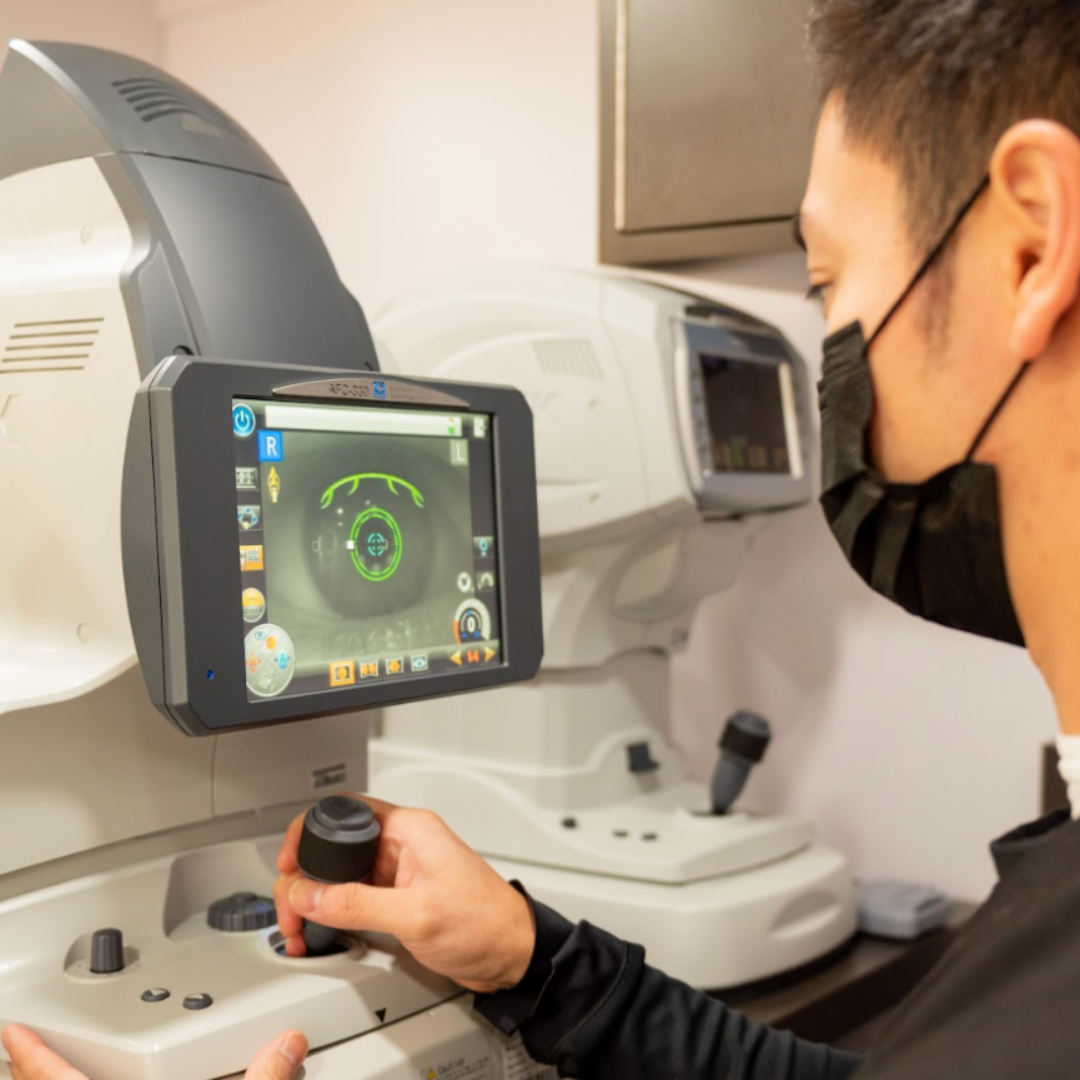
Our certified decentralized post-marketing surveillance professionals ensure high-quality assessments with advanced ophthalmic imaging and diagnostic tools, integrating visual acuity, IOP, OCT, fundus photography, and slit lamp exams. Centralized image upload and built-in quality control protocols deliver consistent, reliable clinical trial post-marketing studies data.
4. Trusted by Leading Sponsors for Long-Term Follow-Up
We handle logistics, scheduling, and documentation for post-market clinical research, so you focus on regulatory compliance. Our team collaborates to identify participant access gaps, re-engage at-risk patients, conduct in-home assessments, and deliver scalable solutions, keeping real-world evidence studies on track.
5. High-Quality Real-World Data Collection and Reporting
With 98% data accuracy and 100% compliance with the clinical trials timeline, we deliver reliable results that meet regulatory standards. Our GCP-aligned documentation and consistent instrumentation ensure no variability due to local providers or logistical challenges, just repeatable data every time.
Why Do Post-Marketing Surveillance Strategies Work?
20/20 Onsite combines convenience, expertise, and technology to improve post-marketing surveillance clinical trials, real-world evidence studies, participant retention, and data integrity, driving successful, patient-centric post-marketing follow-up studies. From eliminating participant travel barriers to achieving retention rates as high as 91% (vs industry standard 70%), our point-of-need assessment methodology delivers measurable results that fulfill regulatory mandates while potentially reducing post-marketing surveillance operational costs.
Revolutionary Post-Marketing Surveillance Outcomes:
- Advanced engagement protocols maintain 91% participant retention rates
- Consistent instrumentation ensures data integrity across all timepoints
- Point-of-need accessibility achieves 95+ Net Promoter Scores
- GCP-aligned documentation maintains 98% regulatory compliance







FAQs: 20/20 Onsite's Post-Marketing and Real-World Evidence Solutions
Click a category to get started
do I book an appointment?
General Information
How is 20/20 Onsite’s ophthalmic clinical trial support different from ‘legacy,’ fixed locations sites?
20/20 Onsite’s support for clinical trials stands apart from traditional, fixed-location sites due to its innovative approach, tailored to enhance participant accessibility, data quality, and trial efficiency. Here’s how 20/20 Onsite is different from legacy sites:
- Point-of-Need Accessibility: Unlike legacy sites, which require participants to travel, 20/20 Onsite brings state-of-the-art, point-of-need ophthalmic assessments directly to trial participants wherever they are—home, work, or even school.
- Patient-Centric Model: By minimizing patient travel and scheduling inconvenience, 20/20 Onsite increases engagement and retention. Our point-of-need clinics help ensure trials stay on track with high participant satisfaction and a 90+ Net Promoter Score.
- Enhanced Enrollment and Diversity: Fixed-location sites often limit access to patients within a specific geography, resulting in the underrepresentation of diverse populations. 20/20 Onsite’s point-of-need clinics enable recruitment from a broader and more diverse demographic, addressing a crucial need in clinical trials to meet enrollment goals effectively.
- Cost Control and Efficiency: Trial delays can cost sponsors millions daily. 20/20 Onsite's point-of-need service model is designed to meet strict timelines, reduce logistical delays, and enable sponsors to manage budgets better.
Overall, 20/20 Onsite offers a streamlined, patient-centered alternative that overcomes the traditional limitations of fixed-location clinical trial sites.
Why are 20/20 Onsite services more cost-effective than ‘legacy’ sites?
20/20 Onsite’s point-of-need service model eliminates the significant barriers that traditional fixed-location sites face, offering pinpoint accessibility and predictable timing by reaching trial participants exactly where they live or work. Reaching participants is especially critical when 80% of clinical trials fail to meet enrollment timelines and 30% of participants drop out, with a cost that can reach thousands of dollars per patient. By delivering assessments directly to participants, 20/20 Onsite reduces selection bias, expands trial reach, accelerates enrollment, and minimizes dropout rates—ensuring trials are completed on time and on budget.
On the other hand, legacy sites rely solely on motivated patients who can travel, a necessity that limits diversity and increases recruitment challenges. 20/20 Onsite’s Net Promoter Score of 90+ reflects high participant satisfaction, boosting retention and engagement throughout the study.
Additionally, legacy sites often struggle to reach underserved or remote populations. However, 20/20 Onsite eliminates this issue by providing point-of-need clinics, delivering high-quality, patient-centric care anywhere needed. This flexibility improves patient satisfaction and enhances trial integrity and data quality, making our service a cost-effective and superior solution for clinical trials.
Is 20/20 Onsite a vendor or a clinical trial site? What role does 20/20 Onsite play in clinical trials?
do I book an appointment?
What are post-marketing surveillance clinical trials?
How do real-world evidence studies differ from traditional clinical trials?
What is decentralized post-marketing surveillance?
How long do post-marketing surveillance clinical trials typically last?
What are post marketing requirements versus post marketing commitments?
What is the difference between Phase IV clinical trials and real-world evidence studies?
Who typically sponsors post-marketing surveillance clinical trials?
What triggers the need for post-approval clinical studies?
What study methodologies do you support?
Services and Capabilities
Does 20/20 Onsite perform non-ophthalmology study assessments?
How can 20/20 Onsite accelerate the success of a specific clinical trial?
Does 20/20 Onsite do recruitment for clinical trials?
Does 20/20 Onsite have a database of potential subjects?
Is 20/20 Onsite better suited for single-visit studies or studies with multi-visits spread over several months?
Is the Principal Investigator and study team provided by 20/20 Onsite?
What types of assessments can be performed in decentralized post-marketing surveillance?
What types of ophthalmic conditions can be monitored in post-marketing surveillance?
How does point-of-care surveillance improve data quality?
How does 20/20 Onsite ensure data consistency across multiple locations?
What medical device post-market surveillance services do you offer?
How do you support ICH E2E pharmacovigilance planning?
What technology platforms support decentralized post-marketing surveillance?
What training do clinical professionals receive for post-marketing surveillance?
Can mobile clinics handle emergency situations during assessments?
What data security measures are in place for real-world evidence studies?
How do you handle multi-language requirements in diverse populations?
What imaging capabilities are available in mobile vision clinics?
Costs and Contracts
How much does the service cost?
What are the details of the 20/20 Onsite standard contract?
How can sponsors reduce costs in post-marketing surveillance clinical trials?
Why do post-marketing surveillance clinical trials have high dropout rates?
What challenges do sponsors face with traditional post-approval clinical studies?
How do real-world evidence studies support market access and reimbursement?
What are the key success metrics for post-marketing surveillance clinical trials?
How do mobile clinics compare cost-wise to traditional site models?
What factors influence pricing for decentralized post-marketing surveillance?
How can sponsors budget for long-term real-world evidence studies?
Are there cost benefits to using point-of-care surveillance for rare diseases?
do I book an appointment?
Regulatory and Technical
What is the Institutional Review Board (IRB) approval process?
Are the 20/20 Onsite systems validated?
How is 20/20 Onsite technical equipment calibrated?
How does real-world data collection support regulatory compliance?
What role do pharmacovigilance post-marketing studies play in surveillance?
What regulatory guidance governs real-world evidence studies?
How do you handle adverse event reporting in real-world evidence studies?
How can post-marketing surveillance support label expansion efforts?
What documentation is required for FDA submissions from real-world evidence studies?
How do you ensure GCP compliance in mobile clinic settings?
What role does the FDA play in post-marketing surveillance oversight?
How are informed consent processes handled in point-of-care settings?
What quality assurance measures are implemented in real-world data collection?
How do you handle protocol amendments in ongoing post-marketing surveillance?
do I book an appointment?
Logistics and Scheduling
When should a site partner with 20/20 Onsite? What is the lead time?
How are the logistics handled?
What are 20/20 Onsite’s scheduling requirements?
Do you have a required minimum number of patients?
Do you only come on-site on certain days/times?
Are 20/20 Onsite services essentially ‘on demand’ (i.e., we just call you, and you go to the patients' homes whenever we want?)
What is the enrollment strategy?
What is the typical timeline for implementing decentralized post-marketing surveillance?
How does point-of-need assessment improve participant retention?
How can sponsors ensure diverse population representation in post-marketing surveillance?
How do you coordinate scheduling across multiple time zones?
What happens if a participant moves during a long-term study?
How do you handle weather delays and emergency situations?
Can assessments be scheduled outside normal business hours?
How do you manage participant communication and follow-up?
What geographic coverage is available for point-of-care surveillance?
How do you handle inventory management for mobile clinics?
What backup plans exist for equipment failures?
People also ask
What goes into an ICH E2E pharmacovigilance plan?
What's the difference between active and passive surveillance?
When does the FDA require postmarketing studies?
Ready to start a conversation about 20/20 Onsite's post-marketing and real-world evidence solutions?
Employers or patients, contact us here.
Copyright © 2026. | Privacy Policy