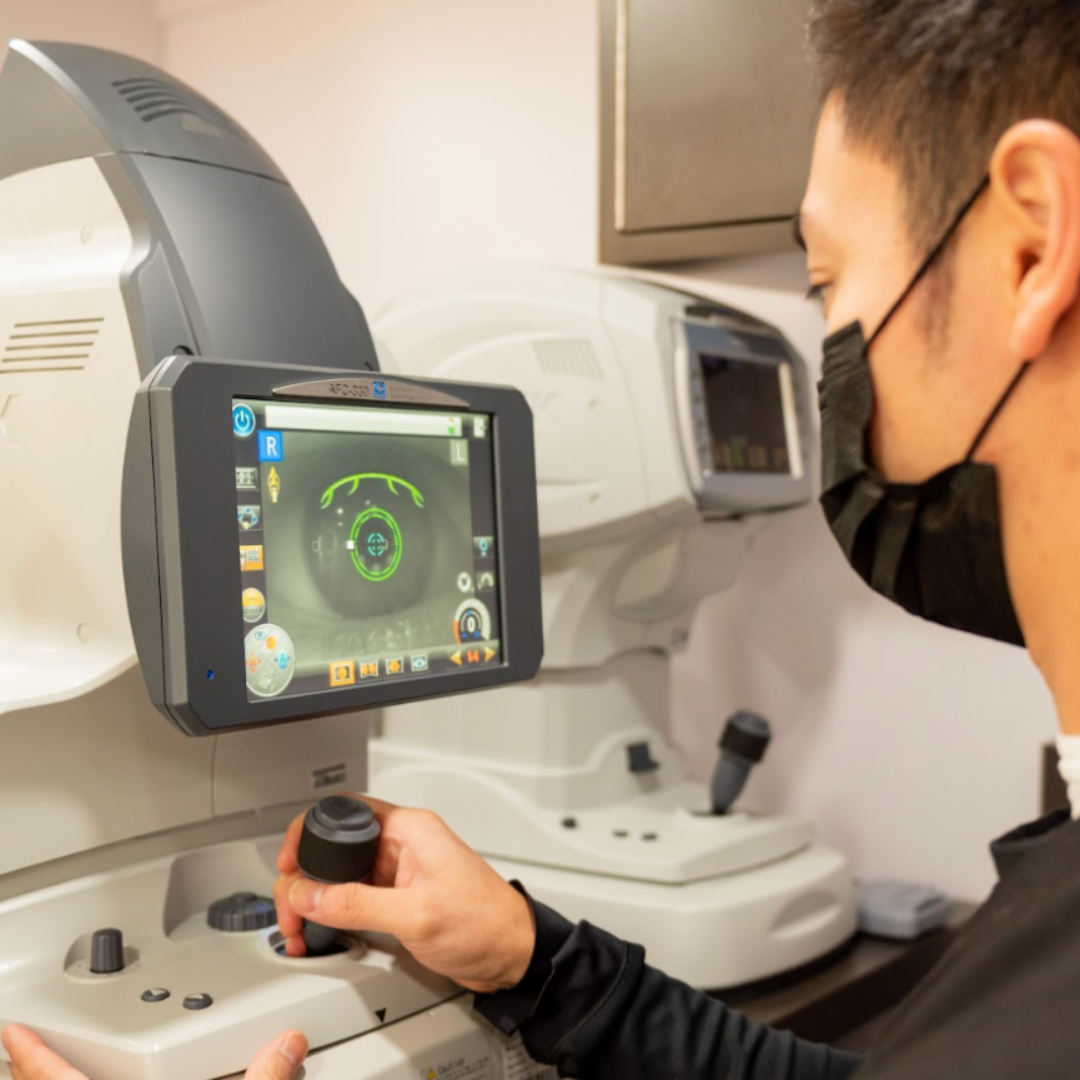
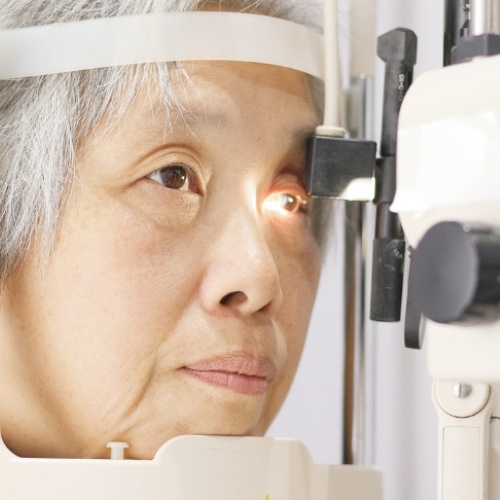
20/20 Onsite specializes in comprehensive clinical trial services for Phase 1,2,3, and post-marketing clinical trials, offering point-of-need clinical research solutions. Our clinical research support services include mobile clinical trials, on-site clinical trial support, and specialized ophthalmic assessments delivered directly to participants.
Clinical Trial Services for Phase 1-3 Studies
Mobile ophthalmic clinical research and clinical trial on-site support services designed to address challenges across all trial phases and ensure reliable protection of ocular endpoints.

Why Phase 1–3 Clinical Trials Fail or Slow Down
Phase 1, 2, and 3 programs can stall due to slow site activation, limited patient access, and uneven protocol execution. Even well-run clinical research sites often face barriers that traditional clinical trial services can’t resolve, leading to delays, missed endpoints, and elevated regulatory risk. Strengthening clinical trial on-site support and patient-centric access points helps reduce these issues and keep timelines on track.
At 20/20 Onsite, our clinical research support services are tailored to each protocol. We align delivery modality, assessment schedule, and onsite oversight to your endpoints and regulatory expectations.
How the right clinical trial services speed up timelines:
-
Site activation: Standardized SOPs, trained staff, and validated equipment accelerate readiness.
-
Patient access: Point-of-need delivery brings assessments to patients, boosting enrollment, diversity, and retention.
-
Protocol variability: Harmonized workflows and QA protect data integrity across sites.
-
Operational complexity: Integrated onsite clinical trial management reduces sponsor/CRO coordination burden.
If you’re evaluating clinical research solutions choose a partner that delivers quality at speed without sacrificing compliance.
Keeping clinical trials on track with patient-centric, point-of-need clinical research solutions
20/20 Onsite delivers comprehensive clinical trial on-site support for Phase 1, Phase 2, and Phase 3 studies through our innovative mobile research model. Our clinical research support services streamline traditional fixed-location sites by bringing state-of-the-art ophthalmic assessments directly to participants, reducing administrative burden for site staff and supporting patient care wherever it’s needed.
Prescreening and Enrollment
We expand access to diverse participants through our clinical research support services and make pre-screening visits easy for patients.
Patient Retention
Ocular Endpoint Protection
Ensure accuracy, consistency, and regulatory confidence with expert ocular endpoint protection through specialized clinical trial support.
Phase 1
Clinical Trial Support with Rapid, Reliable Ocular Assessments
In Phase 1, speed is critical, yet protocols increasingly require specialized safety assessments— including ophthalmic exams— that can delay site readiness and limit participant throughput. Traditional clinical trial site services often struggle to mobilize quickly or support protocol-specific interventions, especially when healthy volunteers aren’t located near academic centers or established research sites. These early-phase challenges can also increase administrative burden for site staff and slow patient enrollment during study start-up.
How Our Clinical Research Support Services Help:
20/20 Onsite brings point-of-need ophthalmic exam directly to your Phase 1 unit or CRO partner site, reducing delays in site activation. Clinical professionals staff our mobile vision clinics with an average of 11+ years of ophthalmic experience, capable of executing protocol-specific assessments (e.g., visual acuity, fundus photography, OCT) with rapid deployment and standardized data capture.
Our clinical research solutions help sponsors meet aggressive timelines while ensuring that early safety signals are captured with precision through specialized Phase 1 clinical trial support.
Learn More About Phase 1 Clinical Trial ServicesPhase 2
Supporting Protocol Refinement with Patient-Centric Clinical Research Solutions
Phase 2 studies often suffer from protocol complexity and increased imaging demands, particularly for ophthalmic endpoints. Patient recruitment slows when eligible participants must travel to limited fixed locations for specialized assessments. This increases screen failure rates and stalls timeline projections in phase 2 clinical trials.
How Our Clinical Trial Services Help:
With full-service mobile vision units and centralized coordination, 20/20 Onsite enables protocol-required ophthalmic imaging through point-of-care clinical research, improving access, retention, and adherence. We support refined endpoint execution (e.g., slit lamp, lens exams, IOP, fundus exams) with quality-controlled data and site-agnostic deployment.
Our clinical research support services team collaborates with clinical operations to identify recruitment bottlenecks and deploy point-of-need assessments strategically to maximize enrollment efficiency without compromising data integrity.
Read Our Phase 2 Case StudiesPhase 3
Reducing Operational Complexity with Scalable Clinical Trial Services
Phase 3 trials demand scalability, consistency, and rigorous adherence to SOPs across dozens or even hundreds of sites. Coordinating ophthalmic assessments at scale becomes increasingly complex, with variability introduced through local providers, inconsistent equipment, and differing levels of expertise. These challenges can impact data management, slow down study start-up, and increase operational burden across therapeutic areas.
How Our Clinical Research Solutions Help:
20/20 Onsite scales with your phase 1, 2, or 3 clinical trials, ensuring uniform, protocol-compliant ophthalmic assessments regardless of location. Our mobile clinical trial units are equipped with standardized instrumentation and can handle complex protocols to ensure data comparability across all visits. With nationwide coverage and a robust data infrastructure, our clinical trial site services help sponsors reduce site burden, ensure audit-readiness, and protect data quality, while improving patient retention through convenient point-of-care clinical assessments.
Whether your trial spans urban hubs or underserved regions, we deliver high-quality assessments through comprehensive clinical trial services at the point of need.
Take a Virtual Tour of Our Mobile Vision Clinic20/20 Onsite: Comprehensive Clinical Trial Services for Phase 1-3
While working with 20/20 Onsite for previous and current studies, we have been able to provide ophthalmological services that were previously not feasible for our clinical units. Doing so has been very successful in providing safety and ophthalmological oversight of study participants – as required by good clinical practices and the sponsor protocol. Dr. David Wyatt, Vice-President and Global Medical Lead, Syneos Health
Proven Results from Our Clinical Trial Services
Point of Need
100%
Screening timelines met across all business operations.
Data Integrity
98%
Data accuracy submission rate.
Patient Retention
91%
Patient retention rate vs industry standard 70%.
Patient Centricity
95+
Patient Net Promotor Score vs industry average NPS of 30-50.







FAQs: 20/20 Onsite's Phase 1-3 Clinical Trial Support Services
Click a category to get started
General Information
What clinical trial services does 20/20 Onsite provide?
How are 20/20 Onsite’s clinical trial site services different from traditional fixed-location sites?
20/20 Onsite’s support for clinical trials stands apart from traditional, fixed-location sites due to its innovative approach, tailored to enhance participant accessibility, data quality, and trial efficiency. Here’s how 20/20 Onsite is different from legacy sites:
- Point-of-Need Accessibility: Unlike legacy sites, which require participants to travel, 20/20 Onsite brings state-of-the-art, point-of-need ophthalmic assessments directly to trial participants wherever they are—home, work, or even school.
- Patient-Centric Model: By minimizing patient travel and scheduling inconvenience, 20/20 Onsite increases engagement and retention. Our point-of-need clinics help ensure trials stay on track with high participant satisfaction and a 90+ Net Promoter Score.
- Enhanced Enrollment and Diversity: Fixed-location sites often limit access to patients within a specific geography, resulting in the underrepresentation of diverse populations. 20/20 Onsite’s point-of-need clinics enable recruitment from a broader and more diverse demographic, addressing a crucial need in clinical trials to meet enrollment goals effectively.
- Cost Control and Efficiency: Trial delays can cost sponsors millions daily. 20/20 Onsite's point-of-need service model is designed to meet strict timelines, reduce logistical delays, and enable sponsors to manage budgets better.
Overall, 20/20 Onsite offers a streamlined, patient-centered alternative that overcomes the traditional limitations of fixed-location clinical trial sites.
Why are 20/20 Onsite services more cost-effective than ‘legacy’ sites?
20/20 Onsite’s point-of-need service model eliminates the significant barriers that traditional fixed-location sites face, offering pinpoint accessibility and predictable timing by reaching trial participants exactly where they live or work. Reaching participants is especially critical when 80% of clinical trials fail to meet enrollment timelines and 30% of participants drop out, with a cost that can reach thousands of dollars per patient. By delivering assessments directly to participants, 20/20 Onsite reduces selection bias, expands trial reach, accelerates enrollment, and minimizes dropout rates—ensuring trials are completed on time and on budget.
On the other hand, legacy sites rely solely on motivated patients who can travel, a necessity that limits diversity and increases recruitment challenges. 20/20 Onsite’s Net Promoter Score of 90+ reflects high participant satisfaction, boosting retention and engagement throughout the study.
Additionally, legacy sites often struggle to reach underserved or remote populations. However, 20/20 Onsite eliminates this issue by providing point-of-need clinics, delivering high-quality, patient-centric care anywhere needed. This flexibility improves patient satisfaction and enhances trial integrity and data quality, making our service a cost-effective and superior solution for clinical trials.
Is 20/20 Onsite a vendor or a clinical trial site? What role does 20/20 Onsite play in clinical trials?
Services and Capabilities
What specific clinical research support services does 20/20 Onsite offer?
Our comprehensive clinical research support services include:
- Point-of-care clinical research assessments
- On-site clinical trial support and coordination
- Mobile clinical trials deployment nationwide
- Pre-screening and participant recruitment
- Safety monitoring and data collection
- Phase 1-3 clinical trial support with specialized protocols
Does 20/20 Onsite perform non-ophthalmology study assessments?
How can 20/20 Onsite accelerate the success of a specific clinical trial?
Does 20/20 Onsite do recruitment for clinical trials?
Does 20/20 Onsite have a database of potential subjects?
Is 20/20 Onsite better suited for single-visit studies or studies with multi-visits spread over several months?
Is the Principal Investigator and study team provided by 20/20 Onsite?
How do 20/20 Onsite mobile clinical trials improve participant experience?
Our mobile clinical trials eliminate travel barriers and scheduling inconveniences. Participants receive professional clinical trial services in comfortable, familiar environments, resulting in higher satisfaction scores and improved retention rates across phase 1, 2, and 3 clinical trials.
What geographic coverage do 20/20 Onsite clinical trial site services provide?
Our clinical trial site services offer nationwide coverage (continental), enabling sponsors to access diverse participant populations previously limited by fixed-location constraints. This expanded reach supports more representative enrollment for clinical research solutions.
Costs and Contracts
How much does the service cost?
What are the details of the 20/20 Onsite standard contract?
How do mobile clinical trials impact study timelines?
Our mobile clinical trials services are designed to accelerate enrollment and reduce delays. Point-of-care clinical research delivery eliminates scheduling bottlenecks common in traditional clinical trial site services, helping sponsors meet critical timeline milestones for phase 1, 2, and 3 clinical trials.
What cost benefits do 20/20 Onsite clinical research solutions provide?
Mobile clinical trials reduce indirect costs associated with participant travel reimbursements, site overhead, and recruitment delays. Our efficient onsite clinical trial management model helps sponsors control budgets while maintaining quality clinical research support services.
How does 20/20 Onsite schedule point-of-care clinical research visits?
Our clinical research solutions include flexible scheduling systems that accommodate participant preferences. Point-of-care clinical research visits are coordinated through our centralized logistics team, ensuring seamless clinical trial services delivery.
Regulatory and Technical
What is the Institutional Review Board (IRB) approval process?
Are the 20/20 Onsite systems validated?
How is 20/20 Onsite technical equipment calibrated?
Logistics and Scheduling
When should a site partner with 20/20 Onsite? What is the lead time?
How are the logistics handled?
What are 20/20 Onsite’s scheduling requirements?
Do you have a required minimum number of patients?
Do you only come on-site on certain days/times?
Are 20/20 Onsite services essentially ‘on demand’ (i.e., we just call you, and you go to the patients' homes whenever we want?)
What is the enrollment strategy?
What strategies help with patient retention across long trials?
20/20 Onsite's point-of-need clinical research model achieves 95+ Net Promoter Scores through convenience-focused clinical trial services. Our mobile clinical trials approach eliminates scheduling conflicts and travel inconveniences that commonly cause participant dropouts. On-site clinical trial management builds stronger participant relationships, while flexible visit scheduling accommodates individual needs throughout phase 1, 2, and 3 clinical trials, significantly improving retention rates.
Ready to start a conversation about 20/20 Onsite's solutions for Phase 1-3?
Contact 20/20 Onsite today to learn how our clinical trial services can accelerate your Phase 1-3 research studies while improving participant experience and data quality.
Employers or patients, contact us here.
Copyright © 2026. | Privacy Policy